Oxides of sulphur
The sulphur content in the fuel determines the content of SOXin the exhaust gas. In the combustion chamber the sulphur present in the fuel is being oxidized into primarily SO2. A much smaller portion, some 3 5% is further oxidized into SO3. Together SO2 and SO3 are called SOX. The cylinder lubrication oil contains substances that serve to neutralize the sulphur and thus prevent damage from sulphuric acid in the engine. Only a very small portion of the SOX is thus neutralized into calcium sulphate and is considered insignificant.
Generally the sulphur content in fuels for land based transportation vehicles is very low and modern power plants are becoming natural gas fuelled or they the exhaust by adding calcium carbonate CaCO3 and hereby form gypsum for the building industry. This makes shipping a big contributor to the global SOX emissions together with oil refineries and older coal and oil fired power plants. The SOX contribution from shipping in 1997 is illustrated in Figure 41.
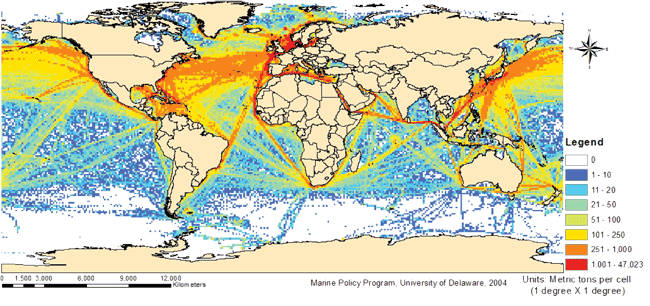
Figure 41 – Global SOX emissions from shipping in 1997. The shipping routes are clearly seen as areas with high SOX concentration. Source: College of Marine and Earth Studies, Delaware.
In 2001 12% of the European sulphur emissions came from shipping and the total could rise to as much as 18% in 2010 and continue to increase as sulphur regulations on land tighten. SOX in mainly known to cause acidic rainfall that damages buildings, lakes and forest areas. In areas with natural low alkalinity, such as parts of Northern Scandinavia, the damage is more pronounced and large areas of forest land have been destroyed along with other adverse effects such as acidification of ground water and damage to aquatic life in fresh water lakes. Limestone buildings are endangered by the acidic rain as they are literally dissolved. In the atmosphere sulphur oxides are washed out rather quickly and have an average lifetime of just two days.
SO2 can be carried with the wind over vast distances and be converted to sulphuric acid, H2SO4, by the humidity in the atmosphere and fall down as rainfall, snow or fog when it encounters the right meteorological conditions. Normal rain has a pH value of about 5 because of a natural content of carbonic acid, but sulphuric acidic rain can have pH values of below 3 – corresponding to vinegar and Coca Cola.
Furthermore SOXis harmful to humans when inhaled by harming the lung functionality and increases the frequency of respiratory infections. In concentrations higher than 500 ppb it can be fatal to weak individuals such as old people. At lower concentrations SO2 can cause chest pain, respiratory problems, eye irritation and increased risk for heart and lung illnesses. SO2 is harmless to healthy people in concentrations below 20 ppb and normal background concentration is about 10 ppb.
CONCAWE (Conservation of Clean Air and Water in Europe), which is the oil companies’ European Association for environment, health and safety in refining and distribution has made a study about sulphur emissions [10]. The study suggest that SOX in the atmosphere have a “global cooling” effect as it is known from volcanic eruptions. The sulphur aerosols in the atmosphere have both direct and indirect negative forcing and thus contribute to counteract the global warming. The direct forcing come from reflection effects of the SOXin the atmosphere and the indirect forcing is due to extra condensation of water vapor around the SOXparticles which bring along increased cloud cover. Clouds have negative radiation forcing i.e. they reflect incoming radiation from the sun.
Aerosols such as SOX have a short lifetime in the atmosphere and are therefore washed out quickly. CO2 and CH4 are long lifetime agents and are slowly removed. The study suggests that the negative forcing from SOX aerosols roughly balances the positive (warming) fording from CO2 from global shipping and if the indirect cooling effect of increased cloud cover is taken into account the SOX emissions from shipping actually leads to a net cooling effect. The study suggests that benefits of the global sulphur emission reduction are counterbalanced by the negative effects the sulphur reduction will have on global warming.
